

The easiest way to tell which is which is to go by electronegativity or by the number of atoms. All other halogens can be either the central or a terminal atom. Compounds of this type are rarely encountered in introductory chemistry, but you should be aware of their existence because they are not uncommon. In these cases hydrogen is said “to bridge” the other atoms or is said to be “bridging”. Examples of this are hydrogen bonding, and a family of compounds called boranes (e. The primary exception to this rule is where H is bonded to more than one other atom. In simple compounds the central atom is the element that appears only once in the formula (example: H 2O, there are two H and only one O, so O is the central atom, even though it is the most electronegative atom). Usually this is the least electronegative atom (example: in CF 4, C is the central atom). Remember that Lewis dot structures only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, they are not useful for compounds (molecules or polyatomic ions) involving transition metal ions except those which have a d 0 or d 10 electronic configuration, for which they do work.Ģ. Our cartoon picture of a molecule is limited, but can give us a rough idea the bonds that hold a polyatomic chemical species together, which is why it is useful. Remember that a Lewis dot structure is an approximation of the actual arrangement of electrons in a molecule or polyatomic ion, much in the same way a cartoon of a cat is an approximation of the actual animal. It is this similarity that allows us to understand the chemistry of complex molecules (especially organic molecules). It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures.
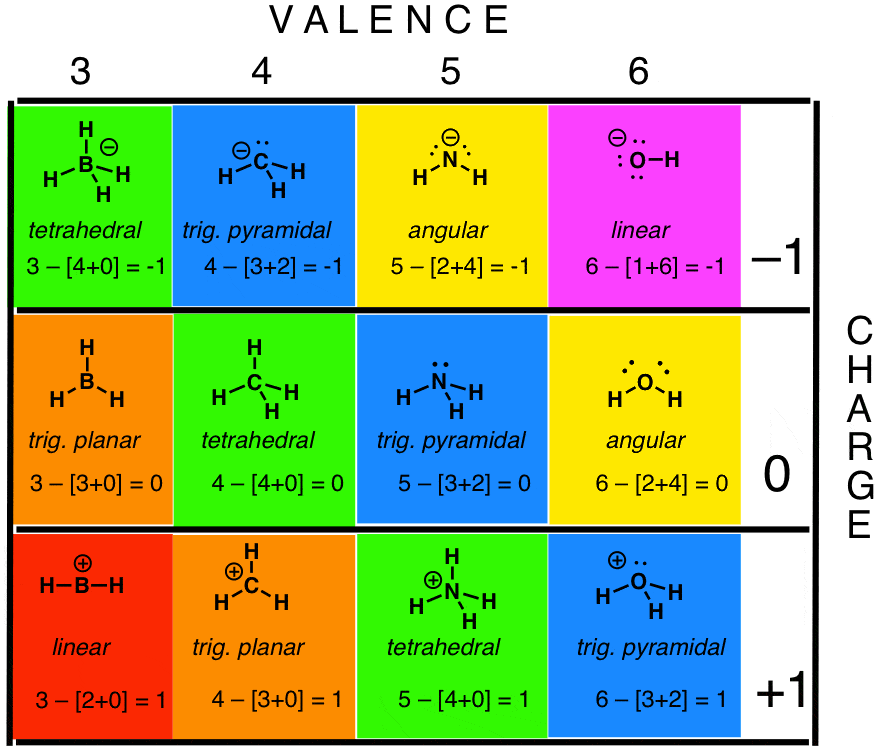
The following is a general procedure for drawing Lewis structures.


 0 kommentar(er)
0 kommentar(er)
